Understanding FDA Recalls of Drugs and Medical Devices
An FDA recall of a drug or medical device is the often voluntary action of a company to correct and/or remove a medicine or product from public circulation because it’s in violation of the laws and regulations that the FDA is charged with enforcing. The nature of the violation may or may not be dangerous. But sometimes they’re deadly. Recalls are an important tool that the government uses to protect the public.
How the FDA Determines Recalls
A defective drug or medical device can be placed on the FDA recall list simply for being in violation of the law or regulations, even if it isn’t known to be dangerous. Although most recalls are voluntary, the FDA is responsible for overseeing how the drug or medical device recall is conducted, assessing the scope of the problem, making sure the violator’s actions are adequate, and classifying the recall according to seriousness. For instance, when evaluating a company’s medical device recall strategy, the FDA considers several factors, including (but not limited to):
- Results of a health hazard evaluation assessing the extent of any harm done, to whom, for how long.
- How well the product being recalled can be identified.
- How obvious it is to the general public that the recalled product is deficient.
- How much the product is used or unused in the market.
- Making sure essential products remain available.
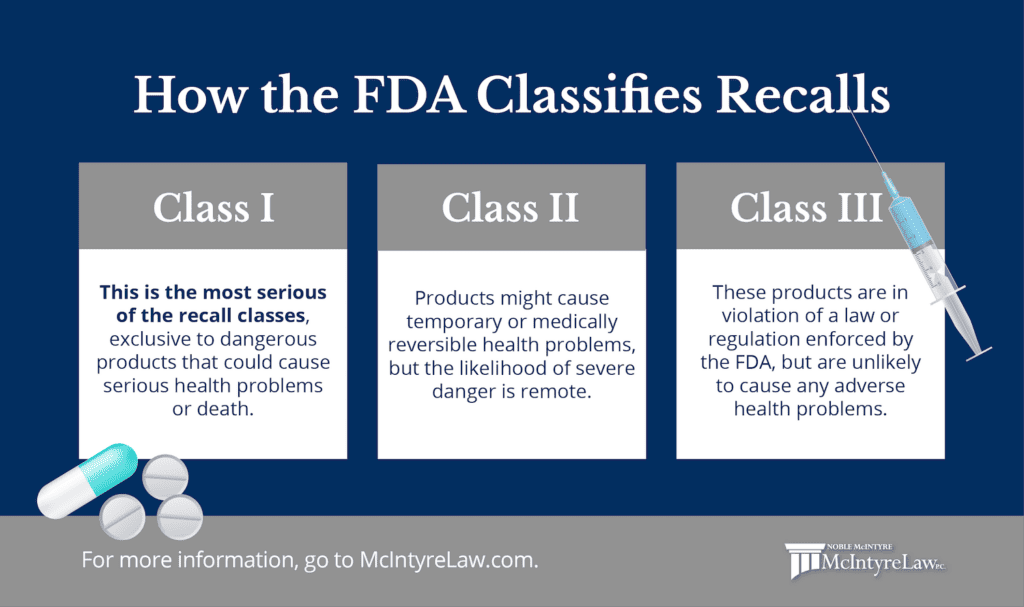
What’s the difference between Class I, II, and III recalls?
FDA recalls fall into one of three medical device and drug recall classes, which reflect the danger posed to the public by the product that’s in violation.
- Class I recall: This is the most serious of the medical device and drug recall classes. This category is for dangerous or defective products that could reasonably be expected to cause serious adverse health problems or even death.
- Class II recall: Products in this category might cause temporary or medically reversible health problems, but the likelihood of severe danger is remote.
- Class III recall: It’s unlikely that products in this category will cause any adverse health problems. They are simply in violation of a law or regulation enforced by the FDA.
Have you suffered after using a drug or medical device on the FDA recall list? Call McIntyre Law to start your case today! (877) 917-5250
Recent FDA Recalls
Not all FDA recalls are given press releases or are posted on the FDA recall list on the agency’s website. However, important information is posted regularly. McIntyre Law believes this to be a vital service to the public. Knowing what medication is recalled or which medical devices are defective can prevent further harm to patients. Here, we list some of the most important recent FDA recalls that the public should know about.
September 2025 FDA Recalls
- BD Alaris Pump Module model 8100 was recalled by BD due to pump performance variations compared to the performance described in the user manual, which could impact infusion delivery.
- Olympus ViziShot 2 FLEX (19G) EBUS-TBNA needles were recalled by Olympus Corporation because device components may detach during procedures.
August 2025 FDA Recalls
- Tandem Diabetes Care t: slim X2 insulin pumps were recalled by Tandem Diabetes Care, Inc. due to a potential speaker-related issue that can trigger an error resulting in a discontinuation of insulin delivery.
- B. Braun Lactated Ringer’s Injection USP 1000 mL / 0.9% Sodium Chloride Injection USP 1000 mL was recalled by B. Braun Medical, Inc., due to particulate matter.
- Max Mobility/Permobil Speed Control Dial Component was recalled by Max Mobility, LLC. due to potential safety and performance concerns.
- DermaRite Industries recalled DermaRite Hand Sanitizers, Cleanser, Skin Protector, and Deodorant due to potential contamination with Burkholderia cepacia.
- DermaKleen, Dermasarra, Kleenfoam, and Perigiene OTC antiseptic lotion soaps, external analgesics, antimicrobial foam soaps, and antiseptic cleansers were recalled by DermaRite Industries, LLC, due to contamination with Burkholderia cepacia.
- Green Lumber Dietary Supplement was recalled by Green Lumber Holding, LLC. due to undeclared prescription drug Tadalafil.
- Unichem Pharmaceuticals Cyclobenzaprine Hydrochloride Tablets USP 10 mg were recalled by Unichem Pharmaceuticals due to device and drug safety concerns related to mislabeling.
July 2025 FDA Recalls
- Tandem Diabetes Care t: slim X2 insulin pumps were recalled by Tandem Diabetes Care, Inc. due to a potential speaker-related issue that can trigger an error resulting in a discontinuation of insulin delivery.
- B. Braun Lactated Ringer’s Injection USP 1000 mL / 0.9% Sodium Chloride Injection USP 1000 mL was recalled by B. Braun Medical, Inc., due to particulate matter.
- Max Mobility/Permobil Speed Control Dial Component was recalled by Max Mobility, LLC. due to potential safety and performance concerns.
- DermaRite Industries recalled DermaRite Hand Sanitizers, Cleanser, Skin Protector, and Deodorant due to potential contamination with Burkholderia cepacia.
- DermaKleen, Dermasarra, Kleenfoam, and Perigiene OTC antiseptic lotion soaps, external analgesics, antimicrobial foam soaps, and antiseptic cleansers were recalled by DermaRite Industries, LLC, due to contamination with Burkholderia cepacia.
- Green Lumber Dietary Supplement was recalled by Green Lumber Holding, LLC. due to undeclared prescription drug Tadalafil.
- Unichem Pharmaceuticals Cyclobenzaprine Hydrochloride Tablets USP 10 mg were recalled by Unichem Pharmaceuticals due to device and drug safety concerns related to mislabeling.
July 2025 FDA Recalls
- Nostrum Laboratories Sucralfate Tablets USP 1 gram were recalled by Nostrum Laboratories, Inc. due to company closure and discontinuation of quality activities.
- Sandoz Cefazolin for Injection, USP, 1 gm vial was recalled by Sandoz, Inc. because vials were incorrectly labelled as Penicillin G Potassium for Injection, but actually contain Cefazolin for Injection.
- Becton, Dickinson and Company Alaris™ and BD Alaris™ Pump Modules were recalled by Becton, Dickinson and Company due to the possibility that they may have been serviced with previously recalled bezel kit assemblies.
June 2025 FDA Recalls
- Sandoz Cefazolin for Injection, USP, 1 gm vial was recalled by Sandoz Inc. due to the potential presence of Penicillin G Potassium Injection vial.
- Newport™ HT70 and HT70 Plus ventilators and certain related service parts were recalled by Medtronic due to device and drug safety concerns regarding a potential defect.
- Zicam® and Orajel™ Cold Remedy Nasal Swabs, Nasal AllClear Swabs, Baby Teething Swabs were recalled by Church & Dwight Co., Inc. due to microbial contamination.
- Amneal Sulfamethoxazole/Trimethoprim Tablets, USP, 400 mg/80 mg were recalled by Amneal Pharmaceutical LLC due to microbial contamination.
May 2025 FDA Recalls
- UNAVY & UMOVY Unavy Acidio Hialuronico (30 caplets/850 mg) and Umovy Acidio Hialuronico (30 caplets/850 mg) were recalled by UMARY USA because the product contains undeclared Dexamethasone, Diclofenac, and Omeprazole.
- GreenWise Pear, Kiwi, Spinach & Pea Baby Food pouches were recalled by Publix due to potential contamination with elevated levels of lead.
- Endurance Boost Dietary Supplement for Male Performance and Energy was recalled by EnShiShiXiangNiShangMaoYouXianGongSi due to undeclared Propoxyphenylsildenafil and Sildenafil.
April 2025 FDA Recalls
- Health Fixer Male Enhancement Dietary Supplements were recalled by Health Fixer due to undeclared Chloropretadalafil, Propoxyphenylsildenafil, and Sildenafil.
- Amneal Pharmaceuticals LLC. Ropivacaine Hydrochloride Injection, USP, 500mg/100mL IV bag was recalled by Amneal Pharmaceuticals LLC. because the product may contain an inert fiber identified as polypropylene fibers from the IV bag.
- Q’Apel Medical 072 Aspiration System was recalled by Q’Apel Medical, Inc. due to distal tip design being outside the scope of 510k clearance.
- Max Mobility/Permobil Speed Control Dial Component was recalled by Max Mobility, LLC. due to an expanded recall from potential safety and performance concerns.
- enVista Aspire™, enVista Envy™ and Certain enVista® Monofocal Intraocular Lenses were recalled by Bausch + Lomb Corporation due to an increased frequency of reports of toxic anterior segment syndrome.
February 2025 FDA Recalls
- CAPS Phenylephrine 40 mg added to 0.9% Sodium Chloride 250 mL in 250 mL Excel Bag was recalled by CAPS due to visible black particulate matter.
- SinuCleanse Soft Tip Squeeze Bottle Nasal Wash System was recalled by Ascent Consumer Products Inc. due to microbial contamination with Staphylococcus aureus (S. aureus).
- Vitality male enhancement dietary supplement capsules were recalled by One Source Nutrition due to undeclared Sildenafil and Tadalafil.
- BD ChloraPrep Clear 1 mL applicator skin preparation product was recalled by BD due to potential fungal contamination under certain environmental conditions allowing the growth of Aspergillus penicillioides.
- ICU Medical POTASSIUM CHLORIDE Inj. 20 mEq and 10 mEq was recalled by ICU Medical due to incorrect overwrap labels stating POTASSIUM CHLORIDE Inj. 10 mEq instead of 20 mEq.
January 2025 FDA Recalls
- Alvogen Fentanyl Transdermal System 25 mcg/h transdermal patches was recalled by Alvogen, Inc. due to potential that patches could be multi-stacked, adhered one on top of the other, in a single product pouch.
- Olympus MAJ-891 endoscope accessory was recalled by Olympus Corporation due to a risk of device contamination that may result from improper reprocessing.
- Provepharm Inc. Phenylephrine hydrochloride Injection, USP, 10 mg/mL was recalled by Provepharm Inc. due to device and drug safety concerns related to potential foreign material.
- Max Mobility/Permobil Speed Control Dial Component was recalled by Max Mobility, LLC. due to potential safety and performance concerns.
December 2024 FDA Recalls
- Astellas Tacrolimus and Tacrolimus Extended-Release capsules were recalled by Astellas Pharma US, Inc. because bottles may contain empty capsules.
- Systane Lubricant Eye Drugs were recalled by Alcon Laboratories due to fungal contamination.
- Par Pharmaceutical Adrenalin® Chloride Solution (EPINEPHrine nasal solution, USP) was recalled by Endo, Inc. because the product is an unapproved drug.
- Fouzee SugarLin Herbal Formula Herbal Dietary Supplement was recalled by Shoppers-Plaza due to undeclared Metformin and Glyburide.
- Force Forever Dietary Supplement was recalled by GNMART Inc. because it contains undeclared diclofenac and dexamethasone.
- Nhan Sam Tuyet Lien Truy Phong Hoan dietary supplement capsules were recalled by Buy-herbal.com due to device & drug safety concerns as an unapproved drug.
November 2024 FDA Recalls
- UMARY Hyaluronic Acid tablets were recalled by MXBBB because the product contains undeclared diclofenac and omeprazole.
- Par Pharmaceutical Clonazepam Orally Disintegrating Tablets, USP (C-IV) was recalled by Endo, Inc. due to mislabeling with the incorrect strength on the carton.
- VitalityVita Marketed as Dietary Supplement was recalled by VitalityVita because it contains undeclared Sildenafil and Diclofenac.
- Boulla Dietary Supplement was recalled by Boulla LLC due to undeclared Sildenafil and Diclofenac.
October 2024 FDA Recalls
- Medtronic MiniMed™ 600 series or 700 series insulin pumps were recalled by Medtronic plc due to device & drug safety defect.
- AK Forte Dietary Supplement was recalled by C&A Naturistics because it is an unapproved drug.
- STASKA Pharmaceuticals Ascorbic Acid Solution for Injection was recalled by STASKA Pharmaceuticals Inc. due to the presence of glass particulates.
- Philips Respironics Trilogy Evo, Trilogy Evo O2, Trilogy Evo Universal, and Trilogy EV300 ventilators were recalled by Philips Respironics, Inc. because the use of in-line nebulizers placed in certain locations may lead to aerosol deposits forming over time on the ventilator flow sensor, potentially causing inaccurate flow measurements affecting therapy.
September 2024 FDA Recalls
- Gilead Veklury (remdesivir) for Injection was recalled by Gilead Sciences, Inc. due to the presence of glass particles.
- Vail-Bon Jie Yang Wan was recalled by 123herbals because it is tainted with dexamethasone and chlorpheniramine.
- Dynacare Baby Powder was recalled by Dynarex Corporation due to potential metal or chemical contaminants.
- BionPharma Atovaquone Oral Suspension, 750 mg/mL was recalled by Bionpharma Inc. due to contamination with Cohnella bacteria.
- Bivona Neonatal/Pediatric and Adult Tracheostomy Tubes were recalled by Smiths Medical because the securement flange of specific lots may tear due to a manufacturing defect.
August 2024 FDA Recalls
- Bloodline Water-Based Tattoo Pigments were recalled by Sierra Stain LLC due to contamination with high concentrations of microorganisms.
- Tandem T:Connect Mobile App for iOS Devices was recalled by Tandem Diabetes Care, Inc. due to rapid depletion of a user’s t:slim X2 insulin pump battery.
- Breas Vivo 45 LS (US Version) Ventilator was recalled by Breas Medical due to potential for short-term elevated levels of formaldehyde exposure.
- B Braun 0.9% Sodium Chloride for Injection USP 1000 mL in E3 containers were recalled by B. Braun Medical Inc. due to potential particulate matter and fluid leakage.
- Baxter Heparin Sodium in 0.9% Sodium Chloride Injection was recalled by Baxter International Inc. due to elevated endotoxin levels.
July 2024 FDA Recalls
- Abbott FreeStyle Libre® 3 sensors were recalled by Abbott due to potential incorrect high glucose readings.
- Healthy Living Migraine Relief Acetaminophen 250mg, Aspirin (NSAID) 250mg & Caffeine 65mg tablets were recalled by Aurobindo Pharma USA, Inc. due to mislabeling.
- Hikma Acetaminophen Injection 1,000 mg per 100 mL (10 mg/mL) 100 mL was recalled by Hikma Pharmaceuticals PLC due to potential presence of Dexmedetomidine HCL Injection inside the overwrap.
June 2024 FDA Recalls
- OxyTote Portable Oxygen Unit was recalled by Western/Scott Fetzer Company due to fire hazard risks from oxygen cylinder failure.
- Flamingo Syrup dietary supplement was recalled by AdamsFlamingoPharma due to undeclared Sildenafil and Tadalafil.
- Ortho Pediatric Femoral Nails were recalled by OrthoPediatrics Corp. due to potential breakage leading to delayed healing.
- ICU Medical Plum 360™ Infusion System was recalled by ICU Medical, Inc. due to issues with the air sensor potentially leading to false air-in-line alarms.
- Aurobindo Pharma Azithromycin Tablets, USP 250 mg and 500 mg were recalled by Aurobindo Pharma USA, Inc. due to potential cross-contamination with another drug.
May 2024 FDA Recalls
- Baxter Sigma Spectrum Infusion Pumps were recalled by Baxter International Inc. due to battery swelling and leakage.
- LifeScan OneTouch Verio® Blood Glucose Test Strips were recalled by LifeScan, Inc. due to potential incorrect glucose readings.
- Teva Pharmaceuticals Topotecan Injection, USP was recalled by Teva Pharmaceuticals USA, Inc. due to possible microbial contamination.
- QuVive™ Dietary Supplement was recalled by NutriSante due to undeclared Sildenafil.
- Stryker LIFEPAK 15 Monitor/Defibrillator was recalled by Stryker Corporation due to power failure issues during patient use.
April 2024 FDA Recalls
- Merck Keytruda (pembrolizumab) injection 100 mg/4 mL was recalled by Merck & Co., Inc. due to the presence of glass particulate matter.
- Philips DreamStation 2 CPAP Machines were recalled by Philips Respironics due to potential foam degradation and health risks.
- GE HealthCare Nuclear Medicine Systems were recalled by GE HealthCare due to detachment risks of detector components.
- Blue Band Instant Dry Yeast was recalled by Lesaffre Yeast Corporation due to possible Salmonella contamination.
- Revolution Tea Green Tea Extract Capsules were recalled by Revolution Tea, LLC due to high levels of lead contamination.
March 2024 FDA Recalls
- Treprostinil 20mg/20mL Injection has been recalled by Endo International, Par Pharmaceutical due to the potential presence of silicone particulate matter.
- CPAP and BIPAP Masks with Magnets have been recalled by Sleepnet Corporation due to the potential for interference with certain medical implants.
- Nimbus Pump System has been recalled by InfuTronix, LLC due to Recall due to a high number (3698) of customer complaints related to the Nimbus Infusion Pump systems.
- Dietary supplements for sexual enhancement have been recalled by Pyramid Wholesale due to undeclared sildenafil and/or tadalafil.
- Vancomycin Hydrochloride for Oral Solution, USP, 250 mg/5mL has been recalled by Amneal Pharmaceuticals, LLC. due to bottles being overfilled.
- Methocarbamol Injection, USP 1000 mg/10 mL (100mg/mL) (Single Dose Vial) have been recalled by Eugia US LLC due to the presence of particulate matter.
February 2024 FDA Recalls
- 1% Tolnaftate Athlete’s Foot Spray Antifungal Spray Liquid has been recalled by Insight Pharmaceuticals due to the presence of benzene.
- Expanded list of Urology and OR room specific kits and trays have been recalled by Cardinal Health due to the potential lack of sterility assurance which could result in a non-sterile product.
- Sustain and Schwinnng brand male enhancement capsules have been recalled by Today the World due to undeclared tadalafil and nortadalafil.
- Arize brand male enhancement capsules have been recalled by Today the World due to undeclared nortadalafil.
- Eye ointment products have been recalled by Brassica Pharma Pvt. Ltd. due to potential lack of sterility assurance.
- MIC* Gastric-Jejunal Feeding Tube Kits have been recalled by Avanos Medical, Inc. due to the potential lack of sterility assurance.
- Mega Soft Universal and Universal Plus Reusable Patient Return Electrodes have been recalled by Megadyne Medical Products, Inc. to restrict use to patients aged 12 years or older.
January 2024 FDA Recalls
- Express Drains have been recalled by Getinge/Atrium Medical Corporation because syringes provided with Express Drains could not be verified to be sterile.
- Vancomycin IV Bags, Phenylephrine IV Bags, and Fentanyl IV Bags have been recalled by Leiters Health due to the potential for superpotent drug.
- Lubricant Eye Drops & Multi-Symptom Eye Drops have been recalled by Kilitch Healthcare India Limited due to potential safety concerns.
- Convenience kits containing saline have been recalled by Aligned Medical Solutions because the product cannot be verified as having the required sterility assurance level.
- Cough syrups have been recalled by Haleon due to microbial contamination.
- Dextroamphetamine sulfate tablets, 30 mg have been recalled by Azurity Pharmaceuticals, Inc. due to mislabeled packaging.
- Neptune’s Fix Elixir, Neptune’s Fix Extra Strength Elixir, and Neptune’s Fix Tablets have been recalled by Neptune Resources, LLC due to undeclared tianeptine.
December 2023 FDA Recalls
- Hospira 4.2% Sodium bicarbonate injection, 8.4% Sodium bicarbonate injection, Atropine sulfate injection have been recalled by Hospira, Inc. due to the presence of glass particulate matter.
- Bleomycin for Injection, USP 15 Units Single Dose ONCO-TAIN™ Glass Fliptop Vial has been recalled by Hospira, Inc. due to the presence of glass particulate matter.
- Americaine Benzocaine Topical Anesthetic Spray has been recalled by Insight Pharmaceuticals due to the presence of benzene.
- Vigabatrin for Oral Solution, USP 500mg has been recalled by InvaGen Pharmaceuticals Inc. due to seal integrity issues that allow for powder leakage from the pouch.
November 2023 FDA Recalls
- Magnum Male Sexual Enhancement XXL 9800 capsule have been recalled by Meta Herbal due to the presence of undeclared Sildenafil.
- Sandimmune (cyclosporine oral solution, USP) Oral Solution 100 mg/mL has been recalled by Novartis Pharmaceuticals Corporation due to crystallization formation.
- TING ® 2% Miconazole Nitrate Athlete’s Foot Spray Antifungal Spray Powder has been recalled by Insight Pharmaceuticals due to the presence of benzene.
- Dietary Supplement with undeclared Sildenafil has been recalled by Noah’s Wholesale, LLC due to presence of the undeclared drug, Sildenafil.
- Vitrakvi ® (larotrectinib) Oral Solution 20 mg/mL in 100mL glass bottles has been recalled by Bayer due to a microbial contamination identified as Penicillium brevicompactum.
- OTC Pain and Fever Reliever for Infants and Kids has been recalled by KinderFarms, LLC due to Acetaminophen instability.
- Multiple brands of Lubricant Eye Drops & Multi-Symptom Eye Drops have been recalled by Kilitch Healthcare India Limited due to potential safety concerns.
- Dr. Ergin’s SugarMD Advanced Glucose Support, Dietary Supplement has been recalled by SugarMDs, LLC due to the presence of undeclared Glyburide and Metformin.
- Multiple brands of dietary supplements with undeclared Diclofenac have been recalled by Botanical-Be due to the presence of the undeclared drug, Diclofenac.
- LEADER OTC Ophthalmic Sterile Drops have been recalled by Cardinal Health, Inc. due to insanitary manufacturing conditions.
- Rugby OTC Ophthalmic Sterile Drops have been recalled by The Harvard Drug Group, LLC dba Major Pharmaceutical and Rugby Laboratories due to insanitary manufacturing conditions.
October 2023 FDA Recalls
- Sodium Bicarbonate Injection, USP, Midazolam in 0.8% Sodium Chloride Injection ELCYS (cysteine hydrochloride Injection), USP has been recalled by Exela Pharma Sciences, LLC due to the potential presence of particulate matter.
- Dietary Supplements with undeclared Diclofenac have been recalled by Botanical-Be due to the presence of the undeclared drug, Diclofenac.
- Over-the-Counter Drug and Medical Device products have been recalled by Family Dollar due to discovery that products were stored outside of labeled temperature requirements.
- Betaxolol Tablets, USP have been recalled by KVK-Tech, Inc. due to the potential presence of Oxycodone HCl tablets.
- 4.2% Sodium Bicarbonate Injection, USP, 1% Lidocaine HCl Injection, USP, and 2% Lidocaine HCl Injection, USP have been recalled by Hospira, Inc. due to the potential presence of glass particulates.
- Iron and Restore brand nasal sprays have been recalled by Biomic Sciences due to potential contamination with Microbacterium spp., Fictibacillus spp., Bacillus spp., and Paenibacillus spp.
September 2023 FDA Recalls
- Brexafemme has been recalled by Scynexis Inc. due to potential cross contamination with non-antibacterial beta-lactam drug substance.
- Test kits have been recalled by Universal Meditech Inc. due to product safety/defect.
- Sucralfate Oral Suspension 1g/10mL has been recalled by VistaPharm due to potential contamination with Bacillus cereus.
- Sandimunne Oral Solution (cyclosporine oral solution, USP) 100 mg/mL has been recalled by Novartis Pharmaceuticals Corporation due to Crystal formation which could potentially result in incorrect dosing.
- TheraBreath Kids Strawberry Splash Oral Rinse has been recalled by Church & Dwight Co., Inc. due to drug safety/microbial contamination.
August 2023 FDA Recalls
- Digoxin Tablets USP, 0.125mg and 0.25mg have been recalled by Marlex Pharmaceuticals, Inc. due to a label mix up.
- Test kits have been recalled by Universal Meditech Inc. due to lack of appropriate premarket clearance or approval which could potentially result in inaccurate test results.
- MSM 5% Solution Eye Drops, MSM 15% Solution Eye Drops, Castor Oil Eye Drops; MSM MIST Drops 5% Solution have been recalled by Dr. Berne’s Whole Health Products due to bacterial and fungal contamination.
- Carina Sub-Acute Care Ventilators have been recalled by Drägerwerk AG & Co. KGaA due to potential foreign material.
- Numerous medical devices, and drug products have been recalled by Inmar Supply Chain Solutions due to the presence of rodent activity at the distribution center and temperature abuse.
July 2023 FDA Recalls
- Tydemy Oral Contraceptive has been recalled by Lupin Pharmaceuticals Inc. due to out of specification results.
- Infusion Pumps have been recalled by Baxter International Inc. due to an increase in false upstream occlusion alarms following the software upgrades.
- Albuterol Sulfate Inhalation Aersol, 90 mcg (200 metered inhalation) has been recalled by Cipla due to failure to deliver the recommended dose.
June 2023 FDA Recalls
- Dronabinol capsules 2.5mg and Ziprasidone Hydrochloride capsules 20mg have been recalled by The Harvard Drug Group LLC due to labeling mix-up.
May 2023 FDA Recalls
- Cough Suppressant, expectorant, nasal decongestant pediatric drops has been recalled by Novis Pr LLC due to device and drug safety mislabeling.
- Wearable Smart Thermometers have been recalled by BearCare Inc. due to potential injuries including skin burns and irritation.
- Pilot Covid-19 At Home Tests have been recalled due to microbial contamination in the liquid buffer solution.
- Various human and animal drug products have been recalled by Akorn Operating Company LLC as a result of bankruptcy.
- Advil has been recalled by Family Dollar due to improper storage temperatures.
April 2023 FDA Recalls
- Fentanyl Buccal Tablets CII have been recalled due to the omission of safety updates in the product medication guide.
- Male Enhancement Capsules have been recalled by Gadget Island Inc. due to undeclared tadalafil and sildenafil.
- Readers in the FreeStyle Libre product family have been recalled due to potential overheating and swelling of the battery.
March 2023 FDA Recalls
- Atovaquone Oral Suspension has been recalled by Camber Pharmaceuticals Inc. due to potential bacillus cereus contamination.
- Alcohol Antiseptic 80% Topical Solution Hand Sanitizer Non-sterile Solution and Isopropyl Alcohol Antiseptic 75% Topical Solution Hand Sanitizer Non-sterile Solution have been recalled due to the presence of methanol.
- Dabigatran Etexilate Capsules, USP has been recalled by Ascend Laboratories LLC due to the detection of N-nitroso dabigatran impurity.
- Life2000 Ventilation System has been recalled by Baxter International Inc. due to the potential for patient oxygen desaturation (low blood oxygen).
- 15% MSM Drops have been recalled by Pharmedica USA LLC as a result of non-sterility.
- Brimonidine Tartrate Ophthalmic Solution, 0.15% has been recalled by Apotex Corp. due to potential lack of sterility.
February 2023 FDA Recalls
- Artificial Eye Ointment has been recalled by Global Pharma Healthcare due to possible microbial contamination.
- Hand sanitizer by nanoMaterials Discovery Corporation has been recalled due to the presence of methanol.
- PrimeZEN Black 6000 male enhancement capsules have been recalled by Volt Candy due to undeclared tadalafil and sildenafil.
- SARS-CoV-2 Antigen Rapid Test Kits have been recalled due to lack of premarket clearance potentially resulting in inaccurate test results.
- Artificial Tears Lubricant Eye Drops have been recalled by Global Pharma Healthcare due to potential microbial contamination.
- TIROSINT ®-SOL (levothyroxine sodium) has been recalled by IBSA Pharma Inc. due to subpotency.
January 2023 FDA Recalls
- Hair & Scalp Spray SPF 30 has been recalled by Edgewell Personal Care due to the presence of benzene.
- Epinephrine bulk API has been recalled by Spectrum Laboratory Products Inc. due to product discoloration.
- CADD Infusion System Infusion Sets for use with CADD pumps has been recalled by Smith’s Medical due to lack of delivery and false NDA alarms.
December 2022 FDA Recalls
- Daptomycin for Injection has been recalled by Accord Healthcare Inc. due to mislabeling.
- Vancomycin Injection has been recalled by Hospira, Inc. due to the presence of visible glass particles.
- After Burn ® Cream and First Aid Kits containing After Burn Cream have been recalled by GFA Production due to contamination with bacillus licheniformis and bacillus sonorensis.
- Quinapril 20 and 40 mg tablets have been recalled by Lupin Pharmaceuticals Inc. due to the presence of nitrosamine impurity, N-Nitroso-Quinapril.
- Over the counter Covid-19 Test have been recalled by Detect Inc. due to the increased chance of false negative results.
November 2022 FDA Recalls
- Sodium Bicarbonate Injection, USP, 8.4%, 50 mEq/50 mL vial has been recalled by Exela Pharma Sciences, LLC due to vial breakage.
- Automated Insulin Delivery System has been recalled by Insulet Corporation due to issues with the omnipod 5 controller charging port and cable.
- Hand sanitizer has been recalled by Adam’s Polishes, LLC due to the presence of methanol.
- Personal Diabetes Managers has been recalled by Insulet Corporation due to battery issues such as swelling, leakage, and extreme overheating.
- Bacterial and viral filters have been recalled by Teleflex Incorporated due to faulty filters.
October 2022 FDA Recalls
- Fresenius Medical Care Hemodialysis Machines have been recalled due to the potential risk of exposure to toxic compounds.
- Aurobindo Pharma USA Inc. Quinapril and Hydrochlorothiazide Tablets have been recalled for two lots in the 20mg/12.5mg dosage due to the detection of N-Nitroso Quinapril impurity.
- Mylan Institutional LLC Octreotide Acetate Injections have been recalled for one lot of the 500 mcg/ml dose due to class particulates in a syringe.
- Whale LLC Mighty Bliss Electric Heating pads have been voluntarily recalled due to product safety concerns and risk of injury.
- Philips Respironics Masks for BiPAP and CPAP Machines have been recalled due to safety issues with magnets.
- Exela Pharma Sciences LLC Sodium bicarbonate Injections, USP, 8.4%, 50 mEq/50 mL Vial, 20-Count Carton, has been recalled due to vial breakage.
- Jiangsu Well Biotech Co., Ltd. COVID-19 Ag Rapid Test Devices have been recalled due to unauthorized, cleared, or approved usage by the FDA.
September 2022 FDA Recalls
- TandemLife LifeSPARC System has been recalled due to the increased risk of critical device failure and software malfunction.
- Golden State Medical Supply, Inc. Atenolol and Clopidogrel Tablets have been recalled for the 25 and 75 mg dosage due to a label mix-up.
- Proper Trade LLC/My Stellar Lifestyle Wonder Pill Capsules have been recalled due to the presence of undeclared Tadalafil.
- Eugia US LLC Acyclovir Sodium Injections have been recalled due to the presence of particulate matter.
- Philips Respironics BiPAP Machines has recalled certain models due to having plastic contaminated with non-compatible material.
- Salon Technologies, Inc. Antica Ocean Citron Hand Sanitizer has been recalled due to the presence of benzene.
- Colgate has recalled certain products sold at Family Dollar Stores due to products being sold outside of temperature requirements.
- Baxter Healthcare Corporation has recalled the Clearlink Basic Solution Set with Duovent due to exposure risks to hazardous or toxic substances.
- Medtronic Endotracheal Tubes have been recalled due to the potential risk of airway obstruction when using specific models.
August 2022 FDA Recalls
- Intera Oncology 3000 Hepatic Artery Infusion Pump has been recalled due to faster than expected flow rates that may impact infusion delivery.
- Hamilton Medical AG Hamilton-C6 Intensive Care Ventilator has been recalled due to Potential Water Ingress that may cause breathing support to stop.
- Medtronic HeartWare HVAD System Batteries have been recalled due to electrical faults that cause battery failure.
- Hospira Propofol Injection Emulsion has a recall of one lot of the medication due to the potential presence of visible particulates.
- Cobalt XT, Cobalt, and Crome Implantable Cardioverter Defibrillators (ICDs) and Cardiac Resynchronization Therapy Defibrillators (CRT-Ds) have been recalled by Medtronic due to devices issuing a short circuit alert and delivering reduced energy shock during high voltage therapy.
- Medtronic Palindrome and Mahurkar Hemodialysis Catheters have been recalled due to catheter hub defects.
- Becton Dickinson Intraosseous Needle Set Kits, Intraosseous Manual Driver Kits, and Intraosseous Powered Drivers have been recalled due to three separate issues that may have caused delayed treatment delivery.
- Haimen Shengbang Laboratory Equipment Co. Ltd. has recalled Viral Transport Media Containers that are not authorized, cleared, or approved by the FDA.
- Plastikon Healthcare Milk of Magnesia, Magnesium Hydroxide/Aluminum Hydroxide/Simethicone Oral Suspension has a voluntary nationwide recall due to microbial contamination.
- Magnesium Citrate Saline Laxative Oral Solution has been recalled by VI-Jon LLC due to microbial contamination.
- Launch Sequence Aphrodisia and Euphoria Capsules have been recalled by Loud Muscle Science, LLC due to the presence of undeclared Tadalafil in the United States and Canada.
- Sangster Energy Supplement has been recalled by distributor RFR, LLC, due to the presence of undeclared sildenafil.
- North American Diagnostics has recalled oral Rapid SARS-CoV-2 Rapid Antigen Test Kits due to products not being authorized, cleared, or approved by the FDA.
July 2022 FDA Recalls
- Edgewell Personal Care Banana Boat Hair & Scalp Sunscreen has been recalled due to the presence of benzene.
- Magnesium Citrate Saline Laxative Oral Solution has been recalled by Vi-Jon, LLC due to microbial contamination.
- Baxter Healthcare Corporation Abacus Order Entry and Calculation Software have been recalled due to an increased risk of medication label errors.
- Ultra Supplement LLC Sustango has been recalled due to the presence of undeclared Tadalafil.
- Family Dollar OTC Medical Products have been recalled due to products being stored outside of temperature requirements.
- Smiths Medical Medfusion 3500 and 4000 Syringe Infusion Pumps have been recalled due to software issues that may impact infusion delivery.
- Vital VIP Vital Honey has been recalled by MKS Enterprise, LLC due to the presence of undeclared Tadalafil.
- Magnesium Citrate Saline Laxative Oral Solution Lemon Flavor has been recalled by Vi-Jon, LLC due to microbial contamination.
- American Contract Systems COVID Test Kit Non-Sterile and Clean Catch Urine Kit have been recalled due to the risk of false results.
- Propofol Injectable Emulsion, USP has been recalled by Hospira due to the potential presence of visible particulates.
- Flow-c and Flow-e Anesthesia Systems by Getinge USA Sales Inc. have been recalled due to cracked or broken suction system power switches.
- Insulin Glargine Injection Pens, 100 units/mL (U-100) by Mylan Pharmaceuticals Inc., have been recalled due to the potential of missing labels on some pens.
June 2022 FDA Recalls
- Morphine Sulfate 30 mg Extended Release and Morphine Sulfate 60 mg Extended-Release by Bryant Ranch Prepack Inc. have been recalled due to a label mix-up.
- GE Healthcare CARESCAPE R860 Ventilator has been recalled due to the early failure of backup batteries.
- Volara System by Baxter Healthcare Corporation has been recalled due to the risk of respiratory distress in ventilated patients during home use.
- HeartWare HVAD System Batteries by Medtronic have been recalled due to battery failure.
- Intraosseous Products by BD have been recalled due to limited or non-functioning intraosseous access and needle stick injuries.
- CVS Magnesium Citrate Saline Laxative Oral Solution Lemon Flavor by Vi-Job LLC has been recalled due to microbial contamination.
- SafeStar 55 Breathing System Filters by Draeger Inc have been recalled due to possible obstructions that may block oxygen flow to patients.
- Artri King Reforzado Con Ortiga Y Omega 3 by Latin Foods Market has been recalled due to the presence of undeclared diclofenac and dexamethasone.
- Medtronic HVAD Pump Implant Kit has been recalled due to a pump weld defect.
- Green Pharmaceuticals Inc SnoreStop NasoSpray has been recalled due to microbial contamination.
- Oral Rapid SARS-CoV-2 Antigen Rapid Test Kit and Joysbio SARS-CoV-2 Antigen Rapid Test Kit (Colloidal Gold) have been recalled by Woodside Acquisitions Inc. due to the lack of authorization, clearance, or approval from the FDA.
- iCast Covered Stent by Atrium Medical has been recalled due to potential balloon or catheter hub separation.
- Milk of Magnesia Oral Suspension and Magnesium Hydroxide,/Aluminum Hydroxide /Simethicone Oral Suspension by Plastikon Healthcare, have been recalled due to microbial contamination.
- Buzzagogo, Inc. Allergy Bee Gone for Kids Nasal Swab Remedy has been recalled due to potential microbial contamination.
- V60 and V60 Plus Ventilators by Philips Respironics have been recalled due to power issues that may cause ventilators to stop with or without alarms.
May 2022 FDA Recalls
- Sara Plus floor lift has been recalled by ArjoHuntleigh Polska due to complaints of smoke and/or flames coming out of the lift.
- Dragonfly OpStar Imaging Catheter has been recalled by Abbott Medical because the marker band may become loose during use.
- Nagrelide Capsules, USP 0.5 mg, has been recalled by Teva Pharmaceuticals USA due to Dissolution Test Failure.
- Avanos Medical Cortrak*2 Enteral Access System has been recalled by Avanos Medicals due to reports of injuries occurring during tube replacement.
- Oral Rapid SARS-CoV-2 Antigen Rapid Test Kit and Joysbio SARS-CoV-2 Antigen Rapid Test Kit (Colloidal Gold) have been recalled by Woodside Acquisitions Inc. due to the lack of authorization, clearance or approval from the FDA.
- Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test (Colloidal Gold) has been recalled by SML Distribution LLC due to the lack of authorization, clearance or approval from the FDA.
- Accula SARS-CoV-2 Test has been recalled by Mesa Biotech, Inc. due to contamination that occurred at the manufacturing facility.
- SyrSpend SF 500mL and 4L have been recalled by Fagron Inc. due to a potential bacterial contamination.
- V60/V60 Plus, and V680 have been recalled by Philips Respironics due to a potential issue with the electrical circuit.
April 2022 FDA Recalls
- DiaTrust COVID-19 Ag Rapid Test has been recalled by Celltrion after being distributed to unauthorized users.
- Harmony Delivery Catheter System has been recalled by Medtronic due to the potential for capsule bond breakage during use.
- Accupril (Quinapril HCl) tablets 10mg, 20mg, 40 mg have been recalled by Pfizer due to the presence of N-Nitroso-Quinapril Content in the product above the Acceptable Daily Intake (ADI) level.
- Insulin Glargine (Insulin glargine-yfgn) Injection has been recalled by Mylan Pharmaceuticals, Inc. because the label is missing on some vials.
- The Mandalorian Hand Sanitizer Ethyl Alcohol 68% and Mickey Mouse Hand Sanitizer Ethyl Alcohol 68% have been recalled by Best Brands Consumer Products, Inc due to the presence of methanol and benzene.
March 2022 FDA Recalls
- LeadCare II, LeadCare Plus, and LeadCare Ultra Blood Lead Tests have been recalled by Magellan Diagnostics, Inc. due to a significant risk of falsely low results.
- IDArubicin Hydrochloride Injection USP has been recalled by Teva Pharmaceuticals after particulate matter, such as silica and iron oxide, was found in the product.
- Milk of Magnesia 2400 mg/30 mL Oral Suspension, Acetaminophen 650 mg/ 20.3mL, and Magnesium Hydroxide 1200mg/Aluminum Hydroxide 1200 mg/Simethicone 120 mg per 30 mL have been recalled by Plastikon Healthcare, LLC due to microbial contamination and failure to properly investigate failed microbial testing.
- Orphenadrine Citrate 100 mg Extended Release (ER) Tablets have been recalled by Sandoz, Inc. due to the presence of a nitrosamine impurity.
- SYMJEPI (epinephrine) Injection 0.15 mg (0.15 mg/0.3 mL) and 0.3 mg (0.3 mg/0.3 mL) Pre-Filled Single-Dose Syringes have been recalled by Adamis Pharmaceuticals Corporation due to potential clogging of the needle.
- Accuretic™ (quinapril HCl/hydrochlorothiazide), quinapril and hydrochlorothiazide, and quinapril HCl/hydrochlorothiazide tablets have been recalled by Pfizer due to the presence of a nitrosamine, N-nitroso-quinapril, above the acceptable daily intake level.
- Certain V60 and V60 Plus ventilators have been recalled by Philips Respironics due to some of these devices containing an expired adhesive.
- STANDARD Q COVID-19 Ag Home Test has been recalled by SD Biosensor due to lack of authorization, clearance, or approval from the FDA.
- COVID-19 Antigen Test (Nasal/Saliva) and COVID-19 IgG/IgM Antibody Test have been recalled by LuSys Laboratories due to the lack of an Emergency Use Authorization, 510(k), or PMA.
- SIGMA Spectrum Infusion Pump with Master Drug Library (Version 8) and Spectrum IQ Infusion System with Dose IQ Safety Software (Version 9) have been recalled by Baxter due to the risk of not alarming for certain events.
- Trimix Formulas F-9, T-105, SB-4, Sermorelin, Sincalide, Hydroxocobalamin, and NAD have been recalled by Olympia Pharmacy due to the products being out of specification.
- TurboHawk Plus Directional Atherectomy System has been recalled by Medtronic due to design similarities shared with another device that was recently recalled for correction.
- Similac PM 60/40 has been recalled by Abbott after an infant tested positive for Cronobacter sakazakii following consumption of the product.
- Sodium Acetate Injection, USP, 400 mEq/100 mL (4 mEq/mL), has been recalled by Fresenius Kabi, USA due to the presence of particulate matter.
- Hand Sanitizer, Isopropyl Alcohol Antiseptic 75%, has been recalled by Tennessee Technical Coatings Corp. due to the product containing methanol.
- 0.9% Sodium Chloride for Injection USP, 250ML in Excel, has been recalled by B. Braun Medical Inc. due to fluid leakage that may cause harm in patients.
February 2022 FDA Recalls
- Arrow-Trerotola Percutaneous Thrombolytic Device (PTD) has been recalled by Arrow International, LLC due to the risk of tip damage or detachment during use.
- RNAstill MTM Specimen Collection Kits have been recalled by BASE10 Genetics because they were marketed and distributed without FDA clearance or approval.
- Certain Similac, Alimentum, and EleCare baby formulas have been recalled by Abbott due to a potential bacterial contamination.
- COVID-19 Direct Antigen Rapid Tests (DART) have been recalled by E25Bio because they are not authorized or approved by the FDA.
- Various drugs and medical devices have been recalled by Family Dollar, Inc. due to the presence of rodent activity at one of their distribution centers.
- bellavista™ 1000 and 1000e Series Ventilators have been recalled by Vyaire Medical Inc. due to issues with software configurations.
- Sure and Brut Aerosol Sprays have been recalled by TCP HOT Acquisition LLC dba HRB Brands due to the presence of benzene.
- STANDARD Q COVID-19 Ag Home Tests have been recalled by SD Biosensor, Inc. due to reports that the test kits were illegally imported into the United States.
January 2022 FDA Recalls
- Polymyxin B for Injection USP, 500,000 Unit per Vial, has been recalled by AuroMedics Pharma LLC due to the presence of particulate matter.
- CovClear COVID-19 Rapid Antigen Test and ImmunoPass COVID-19 Neutralizing Antibody Rapid Test have been recalled by Empowered Diagnostics due to an increased risk of false test results.
- One lot of RevitaDerm Wound Care Gel has been recalled by Blaine Labs Company due to bacterial contamination.
- Certain Trilogy Evo ventilators have been recalled by Philips Respironics due to potential health risks associated with the devices’ sound reduction foam.
- HawkOne Directional Atherectomy System has been recalled by Medtronic Inc. because the catheter tip may break off or separate during use.
- Vaporizer Sevoflurane, Maquet Filling for Flow Family Anesthesia Systems, has been recalled by Getinge due to an increased risk of harmful chemical exposure upon use.
- Semglee® (insulin glargine injection), 100 units/mL (U-100), 3 mL Prefilled Pens, have been recalled by Mylan Pharmaceuticals Inc. (a Viatris company) due to the possibility of missing labels.
- One lot of Senna Syrup, 8.8mg/5mL, has been recalled by Lohxa LLC because of possible microbial contamination.
- Destino Twist 14F (model DST1405525) & Guidestar 14F (model D141103) Steerable Guiding Sheaths have been recalled by Oscor Inc. due to an increased risk of the hub cap and seal detaching during use.
- Metformin HCl Extended-Release Tablets, USP 750 mg, have been recalled by Viona Pharmaceuticals Inc. due to the presence of a potentially harmful impurity.
- WIRION Embolic Protection Device has been recalled by Cardiovascular Systems Inc. due to complaints of the system’s filter tearing or breaking.
- Synergy Cranial and StealthStation S7 Cranial Software has been recalled by Medtronic Inc. due to potential inaccuracies that could result in patient harm.
- Cardiosave Hybrid and Cardiosave Rescue Intra-Aortic Balloon Pumps (IABP) have been recalled by Getinge/Datascope/Maquet due to potential fluid leaks that could cause unexpected pump shutdowns.
- Puritan Bennett 980 Series Ventilator has been recalled by Covidien, LP (part of Medtronic) due to a manufacturing assembly error that may cause the device to stop working properly.
January 2022 FDA Recalls
- Polymyxin B for Injection USP, 500,000 Unit per Vial, has been recalled by AuroMedics Pharma LLC due to the presence of particulate matter.
- CovClear COVID-19 Rapid Antigen Test and ImmunoPass COVID-19 Neutralizing Antibody Rapid Test have been recalled by Empowered Diagnostics due to an increased risk of false test results.
- One lot of RevitaDerm Wound Care Gel has been recalled by Blaine Labs Company due to bacterial contamination.
- Certain Trilogy Evo ventilators have been recalled by Philips Respironics due to potential health risks associated with the devices’ sound reduction foam.
- HawkOne Directional Atherectomy System has been recalled by Medtronic Inc. because the catheter tip may break off or separate during use.
- Vaporizer Sevoflurane, Maquet Filling for Flow Family Anesthesia Systems, has been recalled by Getinge due to an increased risk of harmful chemical exposure upon use.
- Semglee® (insulin glargine injection), 100 units/mL (U-100), 3 mL Prefilled Pens, have been recalled by Mylan Pharmaceuticals Inc. (a Viatris company) due to the possibility of missing labels.
- One lot of Senna Syrup, 8.8mg/5mL, has been recalled by Lohxa LLC because of possible microbial contamination.
- Destino Twist 14F (model DST1405525) & Guidestar 14F (model D141103) Steerable Guiding Sheaths have been recalled by Oscor Inc. due to an increased risk of the hub cap and seal detaching during use.
- Metformin HCl Extended-Release Tablets, USP 750 mg, have been recalled by Viona Pharmaceuticals Inc. due to the presence of a potentially harmful impurity.
- WIRION Embolic Protection Device has been recalled by Cardiovascular Systems Inc. due to complaints of the system’s filter tearing or breaking.
- Synergy Cranial and StealthStation S7 Cranial Software has been recalled by Medtronic Inc. due to potential inaccuracies that could result in patient harm.
- Cardiosave Hybrid and Cardiosave Rescue Intra-Aortic Balloon Pumps (IABP) have been recalled by Getinge/Datascope/Maquet due to potential fluid leaks that could cause unexpected pump shutdowns.
- Puritan Bennett 980 Series Ventilator has been recalled by Covidien, LP (part of Medtronic) due to a manufacturing assembly error that may cause the device to stop working properly.
December 2021 FDA Recalls
- One lot of Clobetasol Propionate Ointment USP, 0.05% in 60 g tubes, has been recalled by Taro Pharmaceuticals U.S.A., Inc. due to the presence of bacteria.
- Nitroglycerin Lingual Spray, 12g bottles, have been recalled by Padagis US LLC because the bottles may not properly dispense the medication.
- Twelve lots of Rompe Pecho CF, Rompe Pecho EX, Rompe Pecho MAX, and Rompe Pecho DM have been recalled by Efficient Laboratories, Inc. due to possible microbial contamination.
- All Edge Pharma, LLC drug products have been recalled by Edge Pharma, LLC due to process issues that could jeopardize the safety and sterility of products.
- Two lots of Lidocaine HCl Topical Solution USP 4%, 50ml, have been recalled by Teligent Pharma, Inc. because of super potency.
- Two lots of Veklury® (remdesivir 100 mg for injection) have been recalled by Gilead Sciences Inc. due to the presence of glass particulates.
- One lot of Enoxaparin Sodium Injection, USP 40mg/0.4 mL, has been recalled by Sandoz, Inc. due to an exposure to high temperatures that may have impacted the product’s effectiveness.
November 2021 FDA Recalls
- Arrow-Trerotola™ Over-The-Wire PTD® Kit Percutaneous Thrombolytic Device: 7FR has been recalled by Teleflex Incorporated due to an increased risk of the orange inner lumen detaching from the device’s basket.
- Four lots of Levetiracetam Injection, USP, have been recalled by Sagent Pharmaceuticals, Inc. due to a lack of sterility assurance.
- American Screening Hand Sanitizer, 8 oz. bottles, has been recalled by American Screening LLC because the bottles resemble beverage containers.
- Ellume COVID-19 Home Tests have been recalled by Ellume due to potential false positive test results.
- 240 lots of SterRx products intended to be sterile have been recalled by SterRx, LLC due to a lack of sterility assurance.
- Various Philips Respironics Ventilators, BiPAP, and CPAP Machines have been recalled by Philips Respironics due to an increased risk of inhaling harmful particles and chemicals during use.
- Custom Convenience Kits containing the Cardinal Health Monoject Flush Prefilled Syringe (0.9% Sodium Chloride) have been recalled by Aligned Medical Solutions due to issues with the device’s plunger.
October 2021 FDA Recalls
- CardioSave Hybrid/Rescue Intra-Aortic Balloon Pumps (IABP) have been recalled by Datascope/Getinge/Maquet due to the risk of battery failure during use.
- ROSA One 3.1 Brain Application has been recalled by Zimmer Biomet due to an issue with the device’s software.
- 8oz Scent Free Hand Sanitizer, Lot G20128A, has been recalled by artnaturals due to the presence of multiple impurities.
- Cardinal Health’s Monoject™ Flush Prefilled Saline Syringes have been recalled by Windstone Medical Packaging dba Aligned Medical Solutions due to a defect with the kit’s plunger mechanism.
- CUBICIN® (daptomycin for injection) 500mg for intravenous use, Lot 934778, has been recalled by Merck due to the presence of glass particles.
- Methocarbamol 500mg has been recalled by Bryant Ranch Prepack due to a mislabelling issue.
- Transseptal Needles and Transseptal Needles with Catheters have been recalled by Cook Medical due to complaints of rust on the products.
- Alinity m SARS-CoV-2 AMP Kit and Alinity m Resp-4-Plex AMP Kit have been recalled by Abbott Molecular, Inc. due to an increased risk of producing “false positive” results.
- Irbesartan and Hydrochlorothiazide Tablets, USP, 150mg/12.5 mg and 300mg/12.5 mg, have been recalled by Lupin Pharmaceuticals, Inc. after they were found to contain high levels of the impurity, N-nitrosoirbesartan.
- Lidocaine HCl Topical Solution 4%, 50ml has been recalled by Teligent Pharma, Inc. due to super potency.
- Procedure Packs containing Smiths Medical NORMOFLO Irrigation Warming Set have been recalled by DeRoyal Industries, Inc. due to potential aluminum contamination.
- ZOOM 71 Reperfusion Catheters have been recalled by Imperative Care Inc. due to an increased risk of the device breaking during use.
- MiniMed™ 600 Series Insulin Pumps have been recalled by Medtronic because they may provide the incorrect dose of insulin to patients.
- MiniMed Remote Controllers used with Paradigm and 508 MiniMed Insulin Pumps have been recalled by Medtronic due to possible cybersecurity risks.
- Lotrimin® AF and Tinactin® spray products have been recalled by Bayer due to the presence of benzene, a known human carcinogen.
September 2021 FDA Recalls
- All Eco-Med ultrasound gels and lotions have been recalled by Eco-Med Pharmaceutical, Inc. due to the risk of bacterial contamination.
- LeadCare II, LeadCare Plus, and LeadCare Ultra Blood Lead Tests have been recalled by Magellan Diagnostics, Inc. due to a risk of false or inaccurate results.
- One lot of Glucagon Emergency Kits have been recalled by Eli Lilly and Company due to a potentially dangerous loss of potency.
- AMSORB PLUS PREFILLED G-CAN 1.0L canisters have been recalled by Armstrong Medical Limited due to the risk of reduced airflow to patients.
- Cordis SUPER TORQUE MB Angiographic Catheter with Radiopaque Marker Bands has been recalled by Cordis Corporation because the marker bands may move or dislodge during use.
- Pipeline Flex Embolization Device and Pipeline Flex Embolization Device with Shield Technology have been recalled by Medtronic due to the risk of fractures or breaks in the device’s delivery system.
- Cefazolin Injection Products have been recalled by IntegraDose Compounding Services, LLC due to concerns about the drug’s sterility.
- NORMOFLO® Irrigation Fluid Warmer and Warmers Sets have been recalled by Smiths Medical ASD, Inc. due to the risk of unsafe exposure to aluminum.
- Level 1® Fast Flow Fluid Warming System, NORMOFLO® Fluid Warmer, and Level 1® Normothermic I.V. Fluid Administration Set have been recalled by Smiths Medical ASD, Inc. due to the potential exposure to toxic levels of aluminum.
- Recirculator 8.0 Disposable Lavage Kit has been recalled by Eight Medical International due to an increased risk of exposure to high levels of aluminum.
- Ruzurgi® (amifampridine) 10 mg tablets have been recalled by Jacobus Pharmaceutical Company Inc. after the discovery of yeast, mold, and bacterial contamination.
- Certain Philips Respironics Ventilators, BiPAP, and CPAP Machines have been recalled by Philips Respironics due to potential health risks upon use.
- Firvanq® (Vancomycin Hydrochloride for Oral Solution), Vancomycin 50 mg/mL Kit has been recalled by Azurity Pharmaceuticals, Inc. after the kits were found to contain the wrong diluent.
- Aminosyn II 15%, an amino acid injection, Sulfite Free IV Solution has been recalled by ICU Medical, Inc. due to the presence of fibers, hair, and other particulate matter.
- Lidocaine HCl Topical Solution 4%, 50ml, has been recalled by Teligent Pharma, Inc. due to super potency.
- LeadCare® Test Kits have been recalled by Meridian Bioscience, Inc. due to a risk of inaccurate readings.
August 2021 FDA Recalls
- Spectrum IQ Infusion Pumps have been issued an Urgent Medical Device Correction by Baxter International Inc. due to potential network connectivity errors.
- Alaris Infusion Pump Module Model 8100 has been recalled by Bio-Medical Equipment Service Co. due to deficiencies in the device’s bezel.
- Monoject™ Flush Prefilled Saline Syringes have been recalled by Cardinal Health due to the risk of air embolisms and other adverse health effects during use.
- Argyle UVC Insertion Tray has been recalled by Cardinal Health due to missing instructions for use.
- Four lots of CHANTIX® (Varenicline) 0.5mg/1 mg Tablets have been recalled by Pfizer due to the presence of N-nitroso-varenicline, a potentially harmful nitrosamine.
- Dose IQ Software Version 9.0.x has been recalled by Baxter Healthcare because of software defects.
- HeartWare Ventricular Assist Device (HVAD) System has been recalled by Medtronic due to an increased risk of adverse events that may result in injury or death.
- INGENIO family of pacemakers and CRT-Ps have been recalled by Boston Scientific due to operational defects that may cause the devices to enter safety mode.
- Sodium Bicarbonate in 5% Dextrose Injection 150mEq per 1,000 mL has been recalled by SterRx, LLC after a microbial contamination was detected.
- Atovaquone Oral Suspension, USP 750 mg/5mL, has been recalled by KVK Tech, Inc. due to defects resulting from exposure to extreme temperatures during shipping.
- Eco-Gel 200 ultrasound gel has been recalled by Eco-Med Pharmaceutical, Inc. due to bacterial contamination.
- V60 and V60 Plus ventilators have been recalled by Philips Respironics due to the risk of reduced oxygen flow to users.
July 2021 FDA Recalls
- GENOSYL DS; Nitric Oxide Delivery System has been recalled by Vero Biotech due to software error.
- Twelve Lots of CHANTIX® (Varenicline) Tablets have been recalled by Pfizer due to N-Nitroso Varenicline content above ADI level.
- NEUTROGENA® and AVEENO® Aerosol Sunscreen Products have been recalled by Johnson & Johnson Consumer Inc. due to the presence of benzene.
- Injectable Semorelin / Ipamorelin 3mg and injectable AOD-9604 3mg have been recalled by Innoveix Pharmaceuticals, Inc. due to a lack of sterility assurance.
- LeadCare II, LeadCare Plus, and LeadCare Ultra Blood Lead Tests have been recalled by Magellan Diagnostics, Inc. due to risk of falsely low results.
- Lyra SARS-CoV-2 Assay (M120) has been recalled by Quidel due to risk of false negative results.
- Angiographic Guidewire Component has been recalled by Medtronic Vascular due to being nonsterile.
- One Lot of Topotecan Injection 4 mg/4 mL (1 mg/mL) has been recalled by Teva Pharmaceuticals due to the presence of particulate matter.
- Limar Hand Sanitizer Packaged in 4 oz Bottles has been recalled by Ardil Commercial due to being packaged in bottles that resemble drink containers.
June 2021 FDA Recalls
- Medical Convenience Kits have been recalled by Avid Medical due to risk of fungal contamination.
- Surgical Procedure Packs have been recalled by DeRoyal Industries due to mislabeled lidocaine.
- Prairie Wolf Distillery Hand Sanitizer Packed in 16.9 Oz. and 20 Oz Bottles has been recalled by Prairie Wolf Spirits, Inc. due to resembling drink containers.
- Durisan Non Alcohol Antimicrobial Hand Sanitizer products have been recalled by Sanit Technologies LLC d/b/a Durisan due to microbial contamination.
- Jelco Hypodermic Needle-Pro Fixed Needle Insulin Syringes have been recalled by Smiths Medical due to skewed odd-number line graduation markings on syringe barrels.
- Philips Bi-Level Positive Airway Pressure (Bi-Level PAP), Continuous Positive Airway Pressure (CPAP), and mechanical ventilator devices have been recalled by Philips due to potential health risks related to the polyester-based polyurethane (PE-PUR) sound abatement foam component in these devices.
- Metformin HCl Extended-Release Tablets, USP 750 mg, have been recalled by Viona Pharmaceuticals Inc. due to the detection of N-Nitrosodimethylamine (NDMA) impurity.
- Innova SARS-CoV-2 Antigen Rapid Qualitative Test has been recalled by Innova Medical Group due to the risk of false test results.
- FiberCel Fiber Viable Bone Matrix (“FiberCel”), Donor Lot Number NMDS210011, has been recalled by Aziyo Biologics, Inc. due to possible post-surgical infection.
- Alaris Infusion Pump Module 8100 Bezel has been recalled by the Biomed Guys due to possible cracked or separated bezel repair posts.
May 2021 FDA Recalls
- SARS-CoV-2 Antigen Rapid Test Kit and Leccurate SARS-CoV-2 Antibody Rapid Test Kit (Colloidal Gold Immunochromatography) have been recalled by Lepu Medical Technology due to risk of false results.
- Alaris Infusion Pump Module 8100 Bezel has been recalled by Step-Har Medical due to possible separated bezel repair posts.
- Medical Convenience Kits have been recalled by Medical Action Industries, Inc. 306 due to risk of fungal contamination.
- VICI VENOUS STENT System and VICI RDS VENOUS STENT System have been recalled by Boston Scientific Corporation due to possible stent migration.
- Durisan Non Alcohol Antimicrobial Hand Sanitizer has been recalled by Sanit Technologies LLC d/b/a Durisan due to bacterial contamination.
- Assurity™ and Endurity™ Pacemakers have been recalled by Abbott (formally known as “St. Jude Medical”) due to the risk of electrical shortages.
- Medically Minded Hand Sanitizer has been recalled by Global Sanitizers due to methanol contamination.
- HeartWare HVAD System Instructions for Use and Patient Manual have been recalled by Medtronic to update safety information.
- Goose Creek Hand Sanitizer and COCO TKO Hand Sanitizer have been recalled by Scentsational Soaps & Candles, Inc. due to the presence of wood alcohol and other contaminants.
- DIBAR Labs Hand Sanitizer has been recalled by Dibar Nutricional S. de R.L. De C.V. due to the presence of wood alcohol, or methanol.
- Levemir®, Tresiba®, Fiasp®, Novolog® and Xultophy® product samples have been recalled by Novo Nordisk due to improper storage temperature conditions.
- 0.5% Bupivacaine Hydrochloride Injection, USP 30 mL and 1% Lidocaine HCl Injection, USP 30 mL, have been recalled by Hospira, Inc. due to product mislabeling.
- Sterile Water for Injection, USP, 100 mL, has been recalled by Hospira, Inc. due to the potential presence of particulate matter.
April 2021 FDA Recalls
- NP Thyroid®, Thyroid Tablets, USP, have been recalled by Acella Pharmaceuticals, LLC due to subpotency.
- Scented Hand Sanitizers have been recalled by Scentsational Soaps & Candles, Inc. due to the undeclared presence of methanol and other contaminants.
- Precise PRO Rx US Carotid System has been recalled by Cordis Corporation due to a risk of separation during use.
- ChloraPrep™ Hi-Lite Orange™ 26 mL Applicator has been recalled by Becton, Dickinson and Company (BD) after a manufacturing error caused defects.
- Bio-Console 560 Extracorporeal Blood Pumping Console has been recalled by Medtronic due to the possibility of electrical failure during use.
- Alaris Pump Bezel Assembly and Alaris Infusion Pumps have been recalled by Tenacore LLC due to possible cracks in bezel repair posts.
- 2001 Tenacore Replacement CareFusion Alaris 8100 bezels have been recalled by Tenacore LLC due to weakened plastic that can disrupt infusion.
- BD Alaris Pump Module has been recalled by CareFusion 303, Inc. due to issues with stuck and unresponsive keys.
- HeartWare HVAD Battery Cables, Data Cables, Adapter Cables and Controller 2.0 Ports have been recalled by Medtronic due to the risk of damaged connector plugs and controller ports.
- Durisan Antimicrobial Hand Sanitizer has been recalled by Sanit Technologies, LLC d/b/a Durisan due to a microbial contamination.
- Evera, Viva, Brava, Claria, Amplia, Compia, and Visia Implantable Cardioverter Defibrillators (ICDs) and Cardiac Resynchronization Therapy (CRT-Ds) have been recalled by Medtronic due to decreased battery life.
- ThermaCor 1200 Rapid Thermal Infusion System disposable sets have been recalled by Smisson-Cartledge Biomedical, LLC due to potential aluminum leaks.
- Valiant Navion Thoracic Stent Graft System has been recalled by Medtronic due to the risk of fracturing or leaking during use.
- Acetaminophen Extra Strength 500 mg Tablets, 100 ct. bottles, have been recalled by A-S Medication Solutions, LLC due to having incomplete drug labels.
March 2021 FDA Recalls
- HVAD Pump Implant Kits have been recalled by Medtronic due to delayed or failed restart after the pump is stopped.
- Spironolactone tablets, 25 mg and 50 mg, have been recalled by Bryant Ranch Prepack due to mislabeling with the incorrect strength.
- Phenylephrine Hydrochloride Injection, USP, 10 mg/mL, has been recalled by Sagent Pharmaceuticals, Inc. due to a lack of sterility assurance.
- Heal the World Hand Sanitizer, in 9.6 oz. bottles, has been recalled by PNHC, LLC d/b/a Heal the World due to risk of ingestion because they resemble water bottles.
- Kodama Intravascular Ultrasound Catheters have been recalled by Acist due to the risk of broken O-ring pieces flushing into arteries during use.
- Valkyrie LTOWB Collection and Administration, Low Titer Type O FWB Transfusion Sets and Low Titer Type O Donor Collection Sets, Fresh Whole Blood Transfusion Sets and Fresh Whole Blood Donor Sets have been recalled by Combat Medical Systems, LLC due to possible bent or broken needles.
- Affinity Pixie Oxygenator and Cardiotomy/Venous Reservoir with Balance Biosurface have been recalled by Medtronic due to possible high levels of endotoxins.
- Durisan Antimicrobial Hand Sanitizer has been recalled by Sanit Technologies LLC d/b/a Durisan due to microbial contamination.
- ChloraPrep 3 mL Applicators have been recalled in every state now by Becton Dickinson and Company due to potential Aspergillus penicillioides contamination.
- Telmisartan Tablets, USP, 20 mg have been recalled by Alembic Pharmaceuticals, Inc. due to the incorrect product strength on labels.
- Acyclovir Sodium Injection, 50 mg/mL, 10 mL and 20 mL vials, has been recalled by Zydus Pharmaceuticals Inc. due to crystallization.
- Guanfacine Extended-Release Tablets 2mg have been recalled by Apotex Corp. due to trace amounts of Quetiapine Fumarate.
February 2021 FDA Recalls
- Unused Valiant Navion Thoracic Stent Graft System has been recalled by Medtronic due to endoleaks, stent fractures, and stent ring enlargement.
- Liko Multirall 200 Overhead Lift has been recalled by Hillrom due to failure to properly attach the Q-link strap lock (also known as the Q-link 1 strap lock) to the S65 hook.
- EMBLEM S-ICD Subcutaneous (Subcutaneous Implantable Cardioverter Defibrillator) System (Models S-ICD A209 and MRI S-ICD A219) has been recalled by Boston Scientific due to risk of short-circuit.
- Adam’s Secret Extra Strength 1500 and Adam’s Secret Extra Strength 3000 capsules have been recalled by Adamssecret.co due to the presence of undeclared sildenafil and/or tadalafil.
- EMBLEM S-ICD Subcutaneous Electrodes (Model 3501) have been recalled by Boston Scientific due to risk of fractures.
- Medfusion 3500 and 4000 Syringe Pumps have been recalled by Smiths Medical due to a risk of medication delivery error.
- ManukaGuard Allercleanse Nasal Spray has been recalled by NDAL MFG INC due to contamination with yeast.
- Enoxaparin Sodium Injection, USP, batches have been recalled by Apotex Corp. due to mislabeling of syringe barrel measurement markings.
January 2021 FDA Recalls
- Deluxe Heat Therapy Massagers, Model 4212, have been recalled by Wahl Clipper Corporation due to risks of overheating.
- Cisatracurium Besylate Injection, USP 10mg per 5mL, has been recalled by Meitheal Pharmaceuticals, Inc. because of a labeling error.
- LOTUS Edge™ Aortic Valve System has been recalled by Boston Scientific Corporation due to issues with the product’s delivery system.
- Ketorolac Tromethamine Injection, USP 30 mg/mL, has been recalled by Fresenius Kabi USA due to the presence of potentially harmful particulate matter.
- Soho Fresh 70% Rubbing Alcohol has been recalled by Essaar Inc. due to methanol contamination.
- Metformin HCl Extended Release Tablets, USP 750 mg, have been recalled by Nostrum Laboratories, Inc. due to unsafe levels of N-Nitrosodimethylamine (NDMA).
December 2020 FDA Recalls
- Chlorhexidine Gluconate Oral Rinse, USP 0.12%, 15mL, has been recalled by Precision Dose, Inc. due to the possible presence of bacteria.
- Paroex® Chlorhexidine Gluconate Oral Rinse, USP 0.12%, has been recalled by Sunstar Americas, Inc. due to a potential bacterial contamination.
- Flexor Check-Flo Introducers and Flexor Tuohy-Borst Side-Arm Introducers have been recalled by Cook Medical because of an increased chance of separation during use.
- IMC Wash-Free Hand Sanitizer has been recalled by Shane Erickson, Inc. DBA Innovative Marketing Consultants due to a potential methanol contamination.
- JET 7 Catheters with Xtra Flex Technology have been recalled by Penumbra due to an increased risk of unexpected death and serious injury.
- Sildenafil 100 mg tablets and Trazodone 100mg tablets have been recalled by AvKare due to a product mix-up that occurred during bottling.
- Anagrelide Capsules, USP 1mg, have been recalled by Torrent Pharmaceuticals Limited due to dissolution test failure.
- Sigma Spectrum Infusion Pumps (V6, V8, and V9) have been recalled by Baxter Healthcare due to issues with unplanned shutdowns during use.
- Regenecare HA Topical Anesthetic Hydrogel has been recalled by MPM Medical, LLC due to a confirmed bacterial contamination.
November 2020 FDA Recalls
- Cook Medical Fixed Core Wire Guide has been recalled by Cook Medical because of a manufacturing error that damaged the device.
- Dexmedetomidine HCL in 0.9% Sodium Chloride Injection has been recalled by Fresenius Kabi USA due to the presence of small amounts of lidocaine.
- Trevo XP ProVue Retriever has been recalled by Stryker Neurovascular due to faulty wires that may break or separate during use.
- Chlorhexidine Gluconate Oral Rinse, USP 0.12%, has been recalled by Lohxa, LLC due to a possible microbial contamination.
- Rashkind Balloon Septostomy Catheters have been recalled by Medtronic Inc. due to quality issues that may cause the device to seperate or fail during use.
- Metformin HCl Extended Release Tablets, USP 500 mg and 750 mg, have been recalled by Nostrum Laboratories due to unsafe N-Nitrosodimethylamine (NDMA) levels.
October 2020 FDA Recalls
- Sigma Spectrum Infusion Pumps (V6, V8 and IQ) have been issued an Urgent Device Correction by Baxter International Inc. after deviations from the specified cleaning methods led to serious injuries.
- Paroex® Chlorhexidine Gluconate Oral Rinse USP, 0.12%, has been recalled by Sunstar Americas, Inc. (SAI) because of a potential bacterial contamination.
- Metformin Hydrochloride Extended-Release Tablets, USP 500 mg and 750 mg, have been recalled by Marksans Pharma Limited due to unsafe levels of N-nitrosodimethylamine (NDMA).
- Smart Care Hand Sanitizer, 0.84 oz. pouches, has been recalled by Ashtel Studios due to packaging that resembles a food or drink container.
- Alaris™ System Pump Module and Pump Module Door Assembly Replacement Kits have been recalled by Becton, Dickinson and Company (BD)/CareFusion 303, Inc. due to stuck or unresponsive keys, which can lead to infusion problems.
September 2020 FDA Recalls
- Cleaner Hand Sanitizer, 500 mL and 1,200 mL, has been recalled by Estado de México, México, DMM VISSION, S.A. de C.V due to the possible presence of methanol.
- Riomet ER, 500 mg per 5 mL, has been recalled by Sun Pharmaceutical Industries because of an N-Nitrosodimethylamine (NDMA) impurity.
- NP Thyroid 15 & NP Thyroid 120 Tablets have been recalled by Acella Pharmaceuticals, LLC due to sub-potent amounts of levothyroxine (T4).
- Alaris™ Syringe and Alaris™ PCA Modules have been recalled by Becton, Dickinson and Company (BD) because of possible display errors that can result in over- or under-infusion.
- Alaris™ System Infusion Pumps have been recalled by Becton, Dickinson and Company (BD) due to four potential hardware malfunctions.
- Alaris™ System PC Unit and PC Unit Front Case Keypad Replacement Kits have been recalled by Becton, Dickinson and Company (BD) due to keys that may become unresponsive or stuck.
- All lots of M Hand Sanitizer Alcohol Antiseptic 80%, 128 oz/3,785 mL, have been recalled by Medek, LLC due to potential methanol presence and sub-potent ethanol levels.
- bio aaa Advance Hand Sanitizer (lot 20DF8307) has been recalled by AJR Trading LLC because of possible methanol contamination.
- Nature-Throid® and WP Thyroid® have been recalled by RLC Labs, Inc. due to sub-potent levels of Liothyronine (T3) and Levothyroxine (T4).
- All lots of Leafree Instant Hand Sanitizer Aloe Vera have been recalled by CorgioMed, LLC because products are labelled as “edible alcohol.”
August 2020 FDA Recalls
- All lots of Always Be Clean Hand Sanitizer and Just Hand Sanitzer that have been labeled to contain methanol have been recalled by Open Book Extracts for being mislabeled.
- Mylan Tranexamic Acid and Amiodarone HCl Injections have been recalled by Mylan N.V. because of vials potentially packaged in incorrect cartons.
- Harmonic Nature Hand Sanitizer has been recalled by Harmonic Nature S. de R.L. Mi due to the potential presence of 1-propanol.
- Zanilast+ Hand Sanitizer Gel has been recalled by Nanomateriales, S.A. de C.V. due to the undeclared presence of 1-propanol.
- V-Klean Hand Sanitizer Gel, Medically Minded Hand Sanitizer Gel, and Protz Real Protection Antibacterial Hand Sanitizer have been recalled by Asiaticon S.A. de C.V. due to the possible presence of methanol and sub-potent ethanol levels.
- Medfusion 3500 and 4000 Syringe Pumps have been recalled by Smiths Medical due to a software error that may cause an over- or under-delivery of medications.
- Florence Morris Antiseptic Hand Sanitizer has been recalled by Grupo Asimex de Mexico S.A. de C.V. due to possible methanol contamination and sub-potent ethanol content.
- Metformin Hydrochloride Extended-Release Tablets USP, 500 mg & 750 mg, have been recalled by Bayshore Pharmaceuticals, LLC due to unsafe levels of the impurity N-Nitrosodimethylamine (NDMA).
- Nuuxsan, Modesa, Assured & Next Hand Sanitizers have been recalled by ALBEK de Mexico S.A. de C.V. due to the undeclared presence of methanol, or wood alcohol.
- Goldenseal Root Powder has been recalled by Maison Terre due to microbial contamination.
- Yacana Hand Sanitizer, 70% alcohol content, has been recalled by Grupo Yacana México S.A.S de C.V. due to potential methanol contamination and sub-potent ethanol content.
- Heparin Sodium Compounded products have been recalled by SCA Pharmaceuticals due to the presence of the undeclared preservative benzyl alcohol.
- SkinGuard24 Hand Sanitizer has been recalled by SG24 LLC due to an indication of methanol presence on the label.
- ChloraPrep™ 3 mL applicators have been recalled by Becton, Dickinson and Company (BD) due to a potential fungal contamination.
- Bersih Hand Sanitizer Gel (fragrance-free) has been recalled by Soluciones Cosmeticas due to the potential presence of methanol.
- Gelbac T Antibacterial Hand Gel has been recalled by Incredible Products S.A. de C.V. due to possible methanol contamination.
- BodyGuard Infusion Pump Systems have had their April 2020 recall updated by CME America due to medication infusion errors.
- DDAVP® Nasal Spray 10 mcg/0.1mL, Desmopressin Acetate Nasal Spray 10 mcg/0.1mL, and STIMATE® Nasal Spray 1.5 mg/mL have been recalled by Ferring Pharmaceuticals US due to higher-than-specified amounts of desmopressin.
- Command Brands Gel AntiBac Instant Hand Sanitizer has been recalled by Roque Plast S.A. de C.V. due to the potential presence of methanol.
- Jaloma Antiseptic Hand Sanitizer has been recalled by Laboratorios Jaloma S.A. de C.V. due to the presence of undeclared methanol, or wood alcohol.
July 2020 FDA Recalls
- Herbacil Antiseptic Hand Sanitizer, 70% alcohol, has been recalled by Broncolin S.A. de C.V due to potential methanol contamination.
- Assured, Blumen & Modesa Hand Sanitizer has been recalled by 4e Brands North America due to undeclared methanol, or wood alcohol.
- Resource Recover & Trading Hand Sanitizer has been recalled by Resource Recover & Trading LLC due to undeclared methanol subpotency ethyl alcohol.
- Born Basic, Scent Theory, et. al. Hand Sanitizer has been recalled by Real Clean Distribuciones SA de CV due to undeclared methanol.
- Shine & Clean Hand Sanitizer Gel has been recalled by Maquiladora Miniara, S.A. de C.V. due to the potential presence of methanol, or wood alcohol.
- Dexmedetomidine Hydrochloride Injection, 200 mcg/50ml, has been recalled by Fresenius Kabi Issues due to cross-contamination of lidocaine.
- Optimus Hand Sanitizer has been recalled by LIQ-E S.A. de C.V. due to potential methanol contamination.
- GlideScope Core One TouchSmart Cable (“OneTouch cable”) has been recalled by Verathon Inc. due to partial or complete loss of image during use.
- bio aaa Advance Hand Sanitizer has been recalled by AAA Cosmetica, S.A. de C.V. due to potential undeclared methanol contamination.
- Bersih Hand Sanitizer Gel Fragrance Free has been recalled by Soluciones Cosmeticas due to potential undeclared methanol contamination.
- Blumen Advanced Hand Sanitizer has been recalled by 4e Brands North America due to the potential presence of methanol, or wood alcohol.
- Metformin Hydrochloride Extended-Release Tablets, 500mg and 1000mg, have been recalled by Lupin Pharmaceuticals Inc. due to an N-Nitrosodimethylamine (NDMA) impurity.
- BodyGuard® Infusion System Administration Sets have been recalled by CME America due to potential overinfusion or underinfusion during use.
- Ovation iX system has been recalled by Endologix® Inc. due to a weak material adjacent to the polymer fill causing leaks.
- Daptomycin for Injection, 500 mg/vial, has been recalled by Mylan Institutional LLC due to the presence of particulate matter.
- All Clean Hand Sanitizer, Moisturizer, and Disinfectant have been recalled by ITECH 361 due to the potential presence of methanol, or wood alcohol.
- Metformin Hydrochloride Extended-Release Tablets USP, 750 mg, have been recalled by Granules Pharmaceuticals Inc. due to an N-Nitrosodimethylamine (NDMA) impurity.
- Arrow AutoCAT®2 and AC3 Optimus® Intra-Aortic Balloon Pump Series have been recalled by Arrow International Inc. due to possible breakdown of motor connector wires.
June 2020 FDA Recalls
- Saniderm Advanced Hand Sanitizer, 70% alcohol content, has been recalled by UVT INC. due to the potential presence of methanol, or wood alcohol.
- TTDeye colored contact lenses have been recalled by Chengdu Ai Qin E-commerce Co. Ltd due to distribution without FDA clearance.
- Children’s Robitussin® Honey Cough and Chest Congestion DM and Children’s Dimetapp® Cold and Cough have been recalled by GSK Consumer Healthcare because of incorrect dosing cups.
- Metformin Hydrochloride Extended-Release Tablets, USP 500mg, have been recalled by Lupin Pharmaceuticals Inc. due to an N-Nitrosodimethylamine (NDMA) impurity.
- Metformin Hydrochloride Extended-Release Tablets, USP 500mg, have been recalled by Apotex Corp. due to an N-Nitrosodimethylamine (NDMA) impurity.
- Metformin Hydrochloride Extended-Release Tablets, USP 500mg and 750mg, have been recalled by Teva Pharmaceuticals USA Inc. due to an N-Nitrosodimethylamine (NDMA) impurity.
- Metformin Hydrochloride Extended-Release Tablets, USP 500mg, have been recalled by Marksans Pharma Limited, India due to an N-Nitrosodimethylamine (NDMA) impurity.
- Metformin Hydrochloride Extended-Release Tablets, USP 500mg and 750mg, have been recalled by Amneal Pharmaceuticals LLC due to an N-Nitrosodimethylamine (NDMA) impurity.
- Biocell textured breast implants and tissue expanders have been recalled by Allergan Aesthetics due to an increased risk of BIA-ALCL.
May 2020 FDA Recalls
- HeartWare HVAD Pump Outflow Graft and Outflow Graft Strain Relief have been recalled by Medtronic due to the risk of breaks and tears during assembly.
- 30-mg, 60-mg and 90-mg NP Thyroid® have been recalled by Acella Pharmaceuticals LLC due to super potency.
- Python Embolectomy Catheters, Bard Embolectomy Catheters, and the OTW Latis Cleaning Catheters have been recalled by Applied Medical due to the risk of catheter tips detaching during use.
- Finasteride Plus 1.25mg capsules have been recalled by MasterPharm LLC due to the presence of an undeclared antihypertensive drug called minoxidil.
April 2020 FDA Recalls
- Langston Dual Lumen Catheters have been recalled by Vascular Solutions Inc. due to the risk of inner catheter separation during use.
- Infusion pumps and infusion sets have been recalled by CME America due to under-delivery of fluid and inaccuracy of pump delivery speed.
- R.E.C.K. (Ropivacaine, Epinephrine, Clonidine, Ketorolac) 50 ml in Sodium Chloride-60 ml BD syringe has been recalled by QuVa Pharma, Inc. due to presence of particulate matter.
- TRUE METRIX® AIR Blood Glucose Meter has been recalled by Trividia Health Inc. because of an incorrect factory-set unit of measure.
- Ketorolac Tromethamine Injection, USP, 30 mg/mL, and Ketorolac Tromethamine Injection, USP, 60 mg/2 mL have been recalled by Fresenius Kabi USA LLC due to presence of particulate matter.
- Ceftazidime for Injection USP (2g) and Dextrose for Injection USP (50 ml) in Duplex® Container have been recalled by Braun Medical Inc. because of elevated levels of high molecular weight polymers.
- Tetracycline HCl Capsules, 250mg and 500mg, have been recalled by Avet Pharmaceuticals Labs Inc. because of low dissolution results.
- Nizatidine Oral Solution 15 mg/mL has been recalled by Amneal Pharmaceuticals LLC because of an NDMA (Nitrosodimethylamine) impurity.
- Methylprednisolone Sodium Succinate for Injection, USP 40mg, 125mg, and 1g have been recalled by Sagent Pharmaceuticals because of an unidentified impurity.
- Hydromorphone HCL Injection, USP CII have been recalled by Hospira Inc. because of the potential for empty or cracked glass vials.
- Imager II 5F Angiographic Catheters have been recalled by Boston Scientific Corporation because the catheter tip could potentially become detached, which could lead to serious injury or even death.
- LeMaitre Over the Wire Embolectomy Catheters have been recalled by LeMaitre Vascular Inc. because of balloon deflation and separation issues.
- All prescription and over-the-counter ranitidine drugs from all manufacturers, including the common brand name Zantac, have been withdrawn entirely from the U.S. market at the request of the FDA.
March 2020 FDA Recalls
- Pipeline flex embolization devices have been recalled by Medtronic because of a fracturing risk.
- STAT-Check and Medline resuscitator bags have been recalled by SunMed Holdings because they were defective.
- Phytonadione injectable emulsion USP, 10 mg/mL, single-dose ampules have been recalled by Dr. Reddy’s Laboratories, Ltd. because the ampules were breaking and shattering.
- Organic kudzu root herbal supplements have been recalled by Mountain Rose Herbs because of a potential salmonella contamination.
- Natural Remedies Active Male capsules have been recalled by the Natural Remedy Store because of the presence of undeclared Tadalafil.
- Ketorolac Tromethamine Injections at 30mg has been recalled by Hikma Pharmaceuticals USA Inc. because of the potential presence of small particulates.
February 2020 FDA Recalls
- Phenytoin Oral Suspension of 125 mg has been recalled by Taro Pharmaceuticals U.S.A., Inc. because of possible underdosing or overdosing of the drug.
January 2020 FDA Recalls
- Rompe Pecho EX, Rompe Pecho CF, and Rompe Pecho MAX liquid have been recalled by Efficient Laboratories, Inc. because of microbial contamination.
- Lamotrigine Tablets of 100mg have been recalled by Taro Pharmaceuticals U.S.A., Inc. because of cross-contamination with another drug substance, Enalapril Maleate.
- Nizatidine Capsules of 150mg and 300mg have been recalled by Mylan N.V. because of an NDMA (Nitrosodimethylamine) impurity.
December 2019 FDA Recalls
- Mirtazapine Tablets of 7.5mg strength have been recalled by Aurobindo Pharma USA, Inc. because of a label error for declared strength.
- Levetiracetam Oral Solution has been recalled by Lannett Company, Inc. because of microbial contamination of Bacillus subtilis.
- Men’s sexual supplements Bull Platinum 30000, Stallion Platinum 30000, Rhino 7 Platinum 30000, Panther Platinum 30000 have been recalled by Motto International Corp because of the presence of undeclared Tadalafil.
November 2019 FDA Recalls
- Blood administration kits have been recalled by B. Braun Medical, Inc. because of a potential leakage at the joint between the blood filter and tubing.
- Ranitidine (commonly known under the brand name Zantac) has been recalled by multiple companies due to a potential presence of N-Nitrosodimethylamine (NDMA) above levels established by the FDA. Given the popularity of the drug, this is one of the most significant medication recalls of the year.
Learn more about this Zantac recall on FDA.gov and Health.Harvard.edu. - Silver Bullet male enhancement capsules have been recalled by Nature’s Rx due to an unapproved active ingredient (sildenafil).
- Up2 libido and dietary supplements have been recalled by Med Man Distribution because the product is tainted with sildenafil.
- Viatrexx sterile injectable products have been recalled by Viatrexx Bio Incorporated because of a lack of sterility assurance.
- Fagron Let’s Gel Kit Convenience Packs have been recalled by Fagron Inc. because they potentially contain microbial contamination in the nonsterile Saturagel.
View more recalls on FDA.gov.

